“I’m just really curious.”
This is the unassuming response you’ll get from Dr. Niels De Jonge if you ask about his prolific research in the fields of physics, electron microscopy, biology, and material science. If you dig a little deeper you’ll uncover the fascinating history of how an easily bored physicist changed the field of in situ microscopy forever.
This is that story.
Niels began his research in experimental physics, focusing on particles generated from nuclear reactions. “I know all about the detection of electrons”, he said, “which is pretty convenient for electron microscopy it turns out.” After finishing his Masters degree from the University of Amsterdam in the Netherlands, Niels began working on his PhD at the Albert Ludwigs University of Freiburg, Germany in an entirely different field – biophysics. This is something of a common trend for Niels, who is constantly exploring new ideas in seemingly unrelated fields of research.
Never one to slow down, Niels finished his PhD and began working right away at the research department of Philips Electronics, the Netherlands, this time focusing his efforts on developing carbon nanotubes for electron microscopy. The theory was that the extraordinary high mechanical strength, high aspect ratio and electrical conductivity of a single nanotube would make it an ideal candid for generating electrons. Niels explored this field for 5 years and eventually ventured out of Phillips to join academia, noting that “carbon nanotubes had a bit too much hype.”
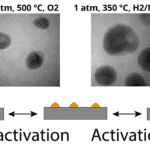
It was around this time that Niels really hit his stride. His passion for innovation and groundbreaking research captured the attention of Oak Ridge National Lab, who brought him on as a strategic hire in 2005. Combining over a decade of research and years of experience in the fields of biology, physics, and microscopy, Niels had a eureka moment. He realized that the fantastic leaps in electron microscopy resolution during the early 2000s came at a cost. Many of the samples that Niels was interested in studying weren’t compatible with the high vacuum environment of the TEM. For example, samples in biological systems like eukaryotic cells, rotoviruses, or proteins, are normally wet, so they become freeze-dried at high vacuum conditions.
The Need for In Situ Liquid Cell Study
Niels glimpsed his calling when he stumbled across a paper by Dr. Frances Ross. “It was an eye-opener for me,” said Niels. The paper outlined the possibility of imaging liquids inside the TEM. Realizing the need to image biological cells in their native, hydrated environment, Niels became convinced. “At some point I just thought, this needs to be done. It’s my job to study samples in liquid.”
But the challenges in making an in situ liquid cell were truly titanic. How would the liquid affect image contrast? How could the liquid be safely contained? Who would fund this research? How would he build the system at all?
This last question was the first one Niels tackled. He was convinced that he could find the engineering know-how and technical prowess needed to build his system by reaching out to custom MEMS chips fabricators and machine shops. “This is what I love so much about the United States. There is such a great innovative spirit,” he said. Within only two years, Niels had his proof of concept. “We eventually took two chips that had thin, electron transparent silicon nitride films, put water between them, and glued them together.” Not the most elegant solution, but this procedure proved his idea was sound in principle.
De Jonge and Partners Develop an In Situ TEM Holder for Liquid Study
Working with Protochips and other engineering companies, the design was refined and one year later, Niels had his first in situ liquid cell holder. He obtained help with the biological side of the research from Dr. Diana Peckys. Now all he had to do was capture images. Oh, and convince the entire academic community that imaging cells in liquid was actually possible.
After experimenting with different contrast-enhancing techniques and spending countless hours exploring electron-liquid interactions, Niels was finally able to achieve his goal – imaging cells in liquid.
“I remember that moment well,” he recalled. “It was a Friday evening, and everyone had already gone home. I was listening to the music of Sherryl Crow on my iPod. I had spent the past 4 months trying to get the perfect image. And then, I did.”
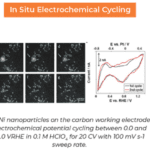
The resulting image is truly spectacular. Proteins decorated with bright, miniscule gold nanoparticles are gently folded within the eurkaryotic cell, faintly reminiscent of stellar nebulae or some other ethereal body. The image not only demonstrated that liquid cell imaging was feasible, the high resolution and excellent contrast hinted that it would become a vital tool for material research and life science research.
The culmination of decades of work, this single image proved Niels right, conclusively, definitively, and permanently.
Since this landmark paper published in PNAS in 2009, Niels has seen in situ liquid cell microscopy transform from a laboratory curiosity to a mainstream tool for academic research. “It’s been really great for Protochips as well,” Niels noted. “They invested a huge amount of resources into making this idea into a commercial product. Now their liquid cell TEM holder is the leader in the field. I’m glad that we are able to work together.”
What’s Next for De Jonge?
“Maybe I’m a bit idealistic, but I want to make an impact,” says the man who has greatly advanced the field of liquid cell microscopy. “I started using Poseidon Select to study cancer-related samples and processes. Cancer is the number one cause of death worldwide, so it would be really great if we could actually cure patients with our research.”
That’s right. Niels de Jonge, a pioneer whose innovative spirit is only outmatched by his scientific curiosity, doesn’t plan to stop with microscopy.
He wants to take on cancer.
Learn more about De Jonge and Protochips’ Poseidon Select in situ TEM holder.