We had the pleasure of interviewing Dr. Peter Benedek, Materials Engineer at Electroflow Technologies, USA. As Dr. Benedek has an extensive background in battery and electrocatalyst materials. In this interview, we aimed to gain insights in the potential of in situ microscopy to bridge the gap between nanoscale and bulk-scale phenomena, ultimately advancing the efficiency and effectiveness of these systems.

Can you tell us a little about your background and your current role at Electroflow?
I began my bachelor’s studies in chemistry and physics with a strong interest in material synthesis. During my master’s thesis, I was introduced to electrochemistry in combination with material synthesis, which sparked my enthusiasm for applied sciences, particularly due to their potential for real-world applications. This led me to pursue a PhD at ETH Zürich, initially focusing on material synthesis, before transitioning into using these methods to explore the properties of battery materials.
As my research progressed, I developed a keen interest in interface science, especially through my work studying nanoparticle surfaces. This research involved extensive spectroscopic analysis, along with simulations to better understand how ion dynamics influence electrochemical behavior at surfaces. By combining these approaches, I gained valuable insights into the interaction between ion dynamics and electrochemistry at interfaces.
Following my PhD, I joined Stanford as a PostDoc, where I shifted my focus to catalysis, studying ‘ideal’ surfaces. In this role, I investigated lithium-mediated nitrogen reduction, a process in which electroplated lithium reacts with nitrogen to form ammonia. A key challenge in this research was understanding how lithium behaves in different electrolytes, which introduced complex interface dynamics. As this was a relatively new field, I also worked on developing a reliable reference electrode and established workflows to better study these systems.
Currently, I am working at Electroflow, where I apply the knowledge and expertise gained from both my PhD and PostDoc. My work involves studying lithium extraction from high-salinity brines using electrochemical methods, specifically through lithium-ion battery technology. This role allows me to combine my skills in battery design, electrolyte development, and material synthesis, offering a dynamic and integrative approach to solving challenges in lithium extraction.

What are your thoughts on incorporating results from nanoscale battery research techniques, such as in situ TEM, as a complement to bulk scale research techniques?
I believe that studies in this area are becoming increasingly feasible as more advanced tools become available for investigating nanoscale processes. The quality of X-ray measurements, electron microscopy, DFT simulations, and other techniques has significantly improved, making it possible to gain more realistic insights at this scale. Personally, I find it crucial to understand electrode interfaces during electrochemical reactions, as these interactions occur primarily at the nanoscale and on surfaces.
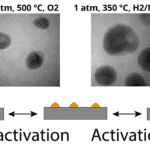
Take lithium-ion batteries, for example: To make their way to the counter electrode, lithium ions, which are predominantly stored in the bulk of the materials, have to first get to the particle surface and then cross from there to the electrolyte. Going through the solid is pretty well understood – there is a large community of scientists that have studied solid state ionics for decades. However, the critical nanoscale phenomena at the surface that allow for the ion transfer are not known. This is why I believe research should focus more on understanding these surface-level interactions during electrochemical processes.
https://pubs.acs.org/doi/abs/10.1021/acsami.9b21470
Which research questions would you like to see pursued using in situ TEM for batteries/electrochemistry?
Studying the surfaces of batteries would be incredibly fascinating, and it’s an area I would like to see explored further. However, surface studies are extremely complex. While I believe there is a wealth of information to be gained from these studies, conducting them properly requires ensuring that impurities do not interfere with the results.
As I mentioned earlier, battery systems are often quite “dirty,” i.e., they contain carbon black, polymeric binders, active materials with complex surface structures, which makes them inherently more complex. If we don’t have a thorough understanding of the system, it’s easy to misinterpret the data from nanoscale studies. While I am eager to see more operando and nanoscale research in this field, I believe it is essential to fully understand the bulk properties of these systems before diving into surface-level investigations.
How would you suggest researchers approach scaling down their battery experiments to the nanoscale?
For me, the first step in this type of research is to create a fully operational cell that mimics the conditions of a bulk-scale reaction. Since I started with a bulk-scale reaction using larger materials, I not only had to scale down the cell but also the materials themselves. This involved synthesizing smaller particles that behaved similarly to the larger ones I had worked with previously.
Once I developed a synthesis method for these smaller particles, I examined their behavior using relatively standardized techniques, such as X-ray diffraction and infrared spectroscopy. After confirming that their properties matched those of the bulk materials, I needed to ensure that their electrochemical behavior was also comparable. I did this by analyzing the electrochemical signal of the smaller particles in the same electrolyte used in my bulk-scale experiments.
My key advice is to approach these studies step by step. It’s crucial to ensure that what is happening at the nanoscale mirrors the behavior at the scale of a commercial battery, and vice versa.
Do you believe it is possible to generate quantitative nanoscale data?
For real quantitative research, we need to consider multiple factors. In bulk-scale reactions, we typically observe several cycles, with each cycle taking 4-5 hours. Attempting this at the nanoscale, especially using electron microscopes, would be time-consuming and expensive. However, most of the significant nanoscale behavior occurs within the first few cycles, which simplifies the process.
If you can capture the results from these initial cycles and demonstrate that they align with the bulk-scale reactions, while incorporating the nanoscale insights from operando studies, it would provide highly valuable information.
What are you hoping to see in the future regarding nanoscale/operando research?
I would love to see a paper that thoroughly covers solid electrolyte interfaces (SEIs) from both a structural and functional perspective. For example, questions like: How does the SEI control species transport? Research that addresses these kinds of fundamental questions would represent a major leap forward for the entire field. Currently, we know very little about these types of interfaces, and gaining a deeper understanding of them could make a significant impact on the progress of battery technology.
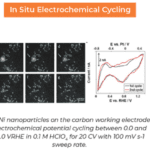
An example of SEI research performed by Dr. Ahmed M. Abdellah et al.
Thanks to Dr. Benedek for sharing his experience in the batteries/electrocatalysis field.
To learn more about research using our systems at the nanoscale for batteries, click here!
If you want to know more about in situ work with our newest Triton AX system, click here!
Want to know a bit more about battery research at the nanoscale? Read this review article:
Electron microscopy and its role in advanced lithium-ion battery research