The chemical reactions occurring at the surface of nanoscale catalysts can drastically alter the performance and reliability of those catalysts. With in situ microscopy, you can change environmental conditions of these reactions and directly observe the chemical, structural, and morphological changes underpinning the catalysis reaction with atom-by-atom precision.
Atmosphere AX: Gas and vapor high temperature environment
Image nanoparticle catalyst reactions occuring at high temperature and pressures while maintaining atomic resolution.
Click here to learn more about our gas cell solution
Poseidon AX: Liquid, electrochemical and liquid heating in situ studies
Image real time electrocatalysis reactions, synthesis of catalyst particles as well as catalyst interactions in liquid
Click here to learn more about our liquid cell solution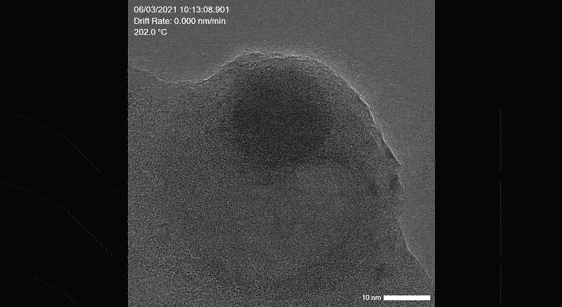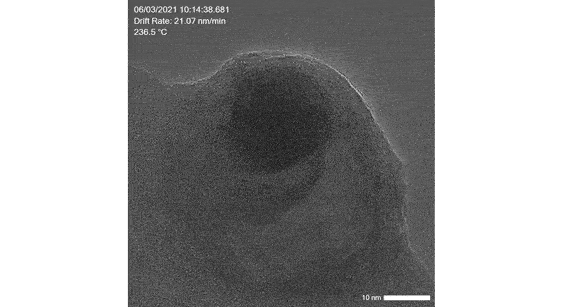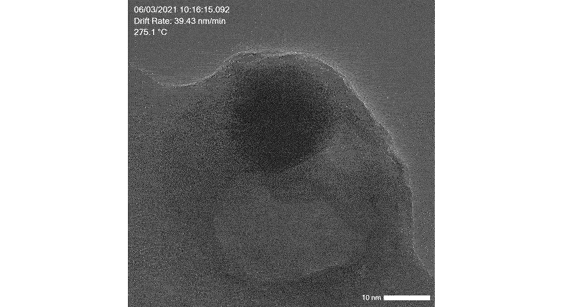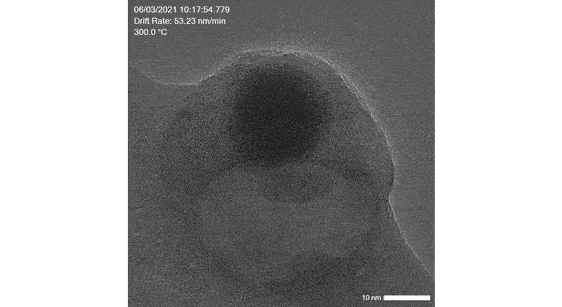
Fusion AX: Study high temperature heating, electrical and electrothermal in vacuum
Heat catalysts to reaction conditions while observing chemical and structural changes in situ.
Click here to learn more about our in situ heating and biasingFeatured Papers
-
News
Sintering Mechanism of Pt...Sep. 17, 2024 -
News
Platinum-Based Nanowires ...Aug. 27, 2024 -
News
An In Situ Investigation ...Aug. 13, 2024 -
News
In Situ TEM Study of Rh P...Jul. 9, 2024 -
News
Investigating Palladium N...Jun. 18, 2024 -
News
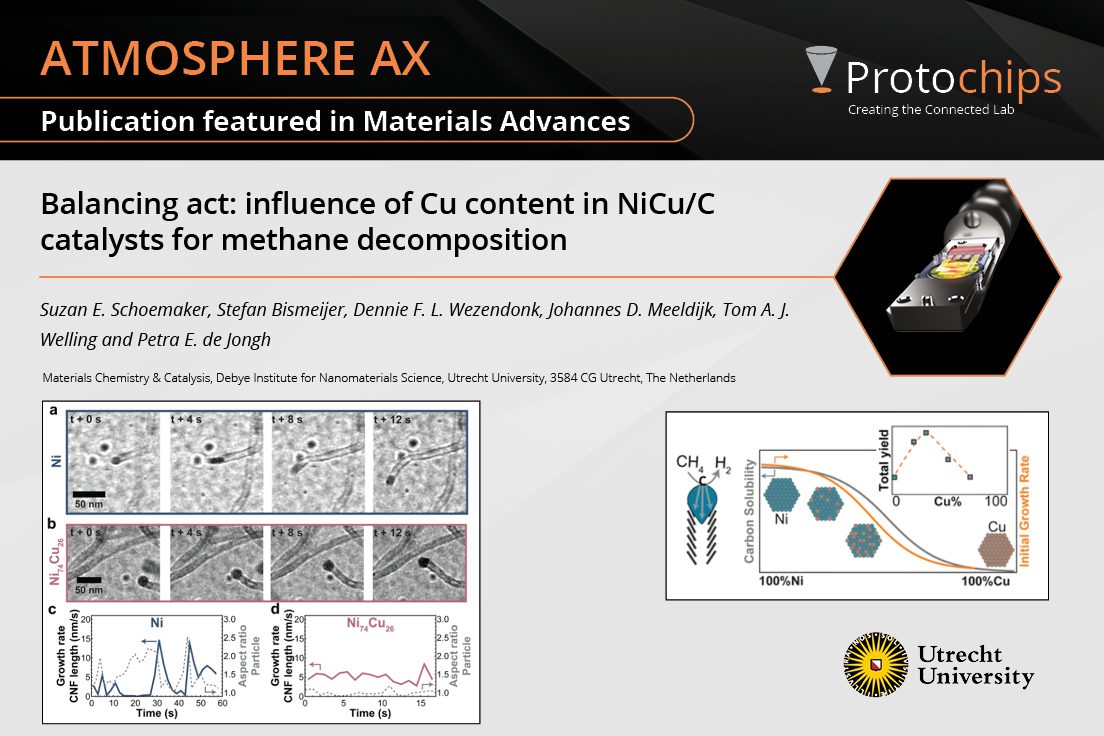
Balancing act: influence ...May. 21, 2024 -
News
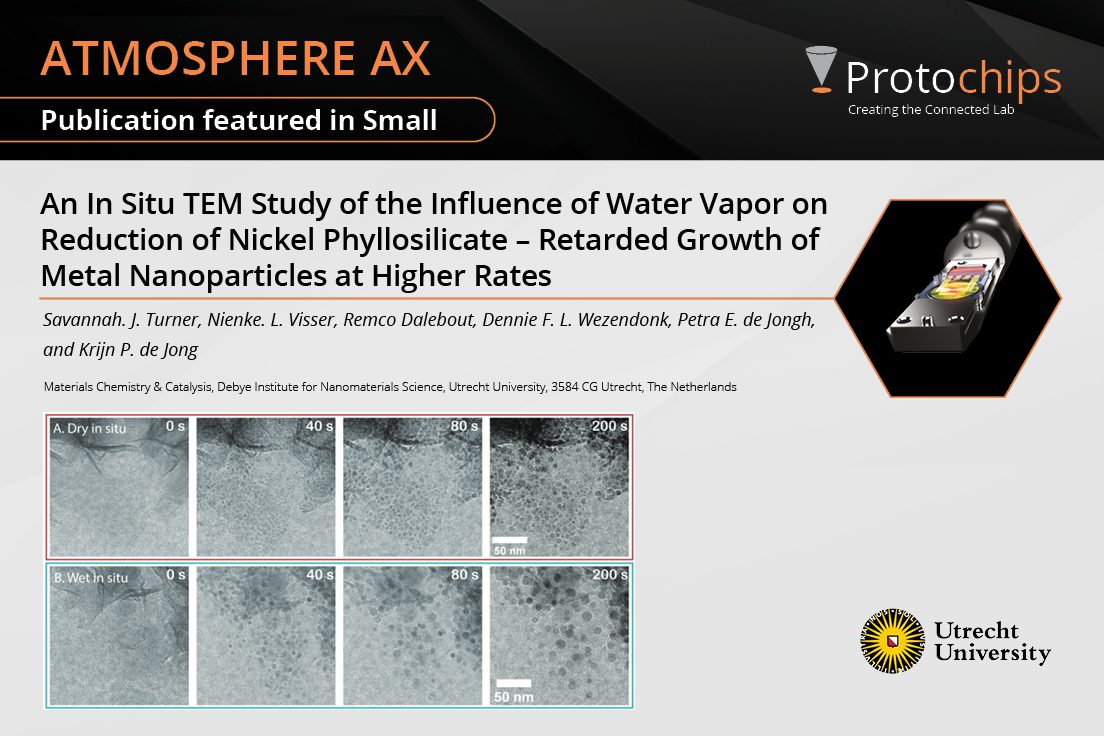
An In Situ TEM Study of t...May. 7, 2024 -
News
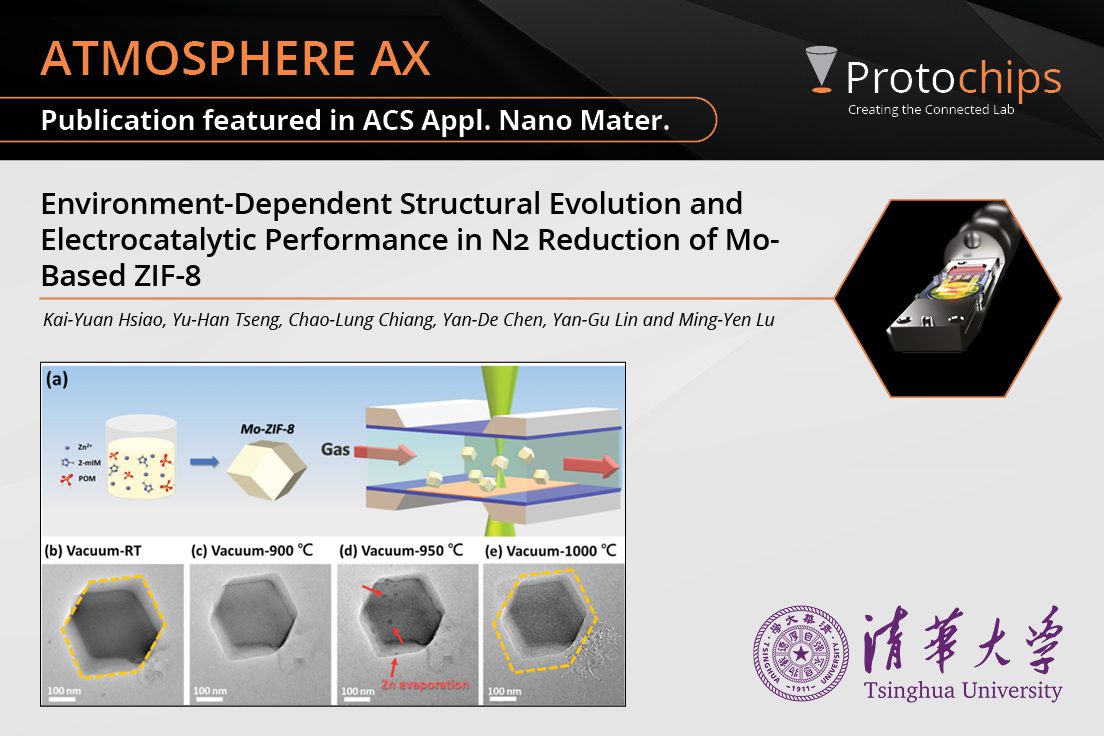
Environment-Dependent Str...Apr. 16, 2024 -
News
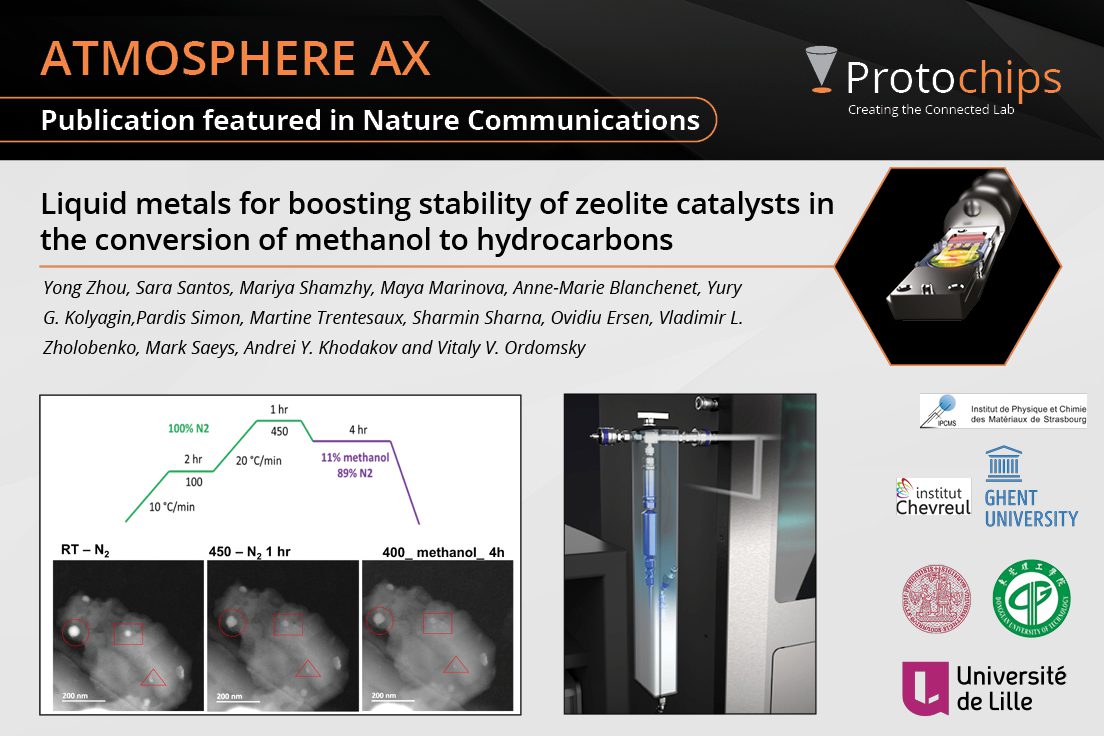
Liquid metals for boostin...Apr. 2, 2024 -
News
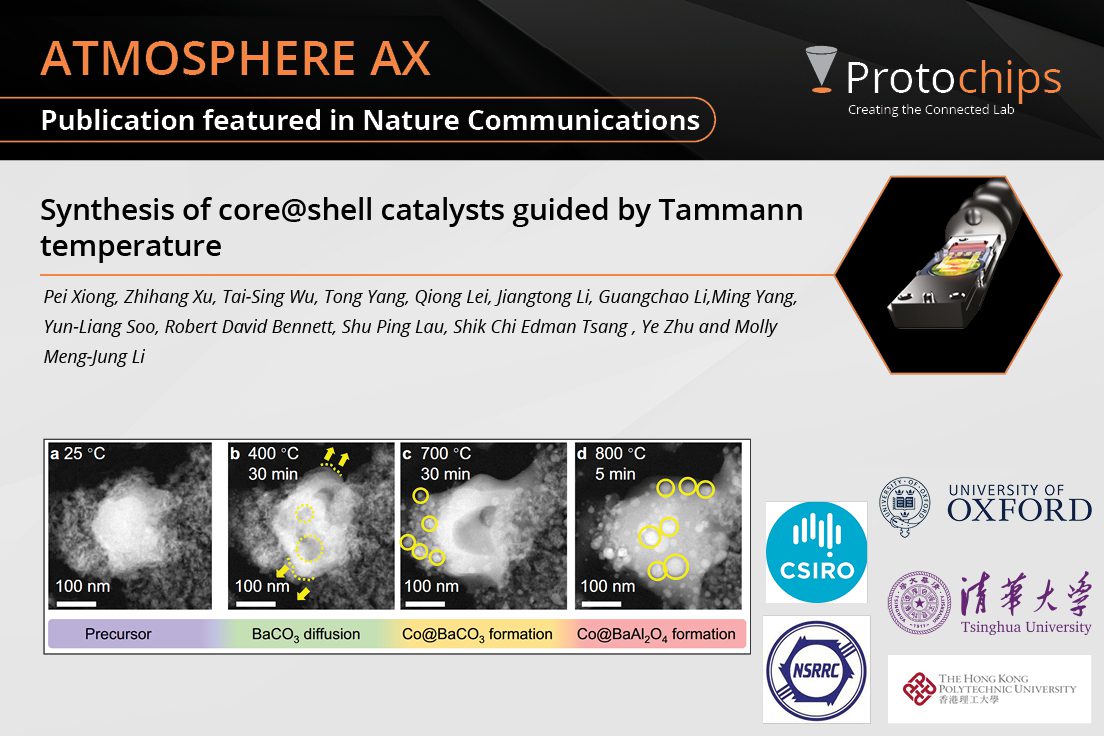
Synthesis of core@shell c...Mar. 5, 2024
What have our users done in the field? Read our summaries here!
-
Heterogeneous Catalysis One Pager
Download the one pager that shows how Atmosphere AX can be used to study heterogeneous catalysts with in situ electron microscopy.
-
Using Protochips AXON Software for Tracking Electron Flux and Cumulative Dose
In this paper, we take a look at how AXON Dose tracks the electron flux and cumulative dose, and why this might be useful for all experiments.
-
Novel Catalyst Structure Dynamics Captured at the Atomic Scale Using the Protochips Atmosphere Gas E-cell System
In this summarized paper, the Atmosphere system was used to better understand CeO2 catalyst behavior under reaction conditions through a collaborative study between researchers at University of Pennsylvania and University of Michigan.
-
Visualizing Hydrogen Absorption in Palladium Thin Films using In Situ Environmental TEM
In this summarized paper, researchers in the Materials Science department at the University of Manchester performed in situ hydrogen absorption experiments on a thin film of Pd using the Atmosphere system.
-
In Situ Few Layer Graphene Etching Using Iron Nanoparticles
In this summarized paper, researchers at DSI-IPCMS-CNRS/University of Strasbourg, (France, G. Melinte, Dr. S. Moldovan and Prof. O. Ersen) used the Atmosphere system to visualize the FLG etching process under relevant reaction conditions.
-
In situ environmental study of perovskite-noble metal catalysts for automotive exhaust control using a Cs corrected TEM
In this summarized paper, Xiaoqing Pan group at University of Michigan performed in situ high-temperature reduction experiments on a CaTi0.95Rh0.05O3 catalyst sample using the Atmosphere system.
-
Dynamic Imaging and Elemental Analysis of Nanostructures in Liquid
In this paper, written by Protochips, nanometer resolution elemental mapping of nanostructures in solution was demonstrated using the Poseidon system.
Videos
Watch dynamic behavior of real samples in situ.
This informational video shows how the Atmosphere AX system can be used to image heterogeneous catalysts within the electron microscope. The Atmosphere AX system is a gas phase electron microscopy system that consists of a cell made out of two MEMS devices (E-chips). In this video, examples from literature will be shown on what type of research, within the field of Heterogeneous Catalysis, can be performed.
With ongoing oxidation, you are seeing the spreading of a porous oxide structure resulting from so called “Kirkendall effect”, which is visualized in the atomic level of resolution during the heating ramp. The integrated metadata to was used to plotting and analyzing trends.
Copper catalyst particle analyzed in the Protochips Atmosphere in situ TEM system while utilizing the AXON Machine Vision Software platform for on-the-fly stabilization, real-time data integration, and data analysis and video generation. Read more about Atmosphere and AXON on our website: www.protochips.com’
This real-time video shows two gold nanoparticles that are on the surface of larger iron oxide particles at 900° C. At this temperature the gold is very mobile, and the two particles coalesce into one larger nanoparticle. This demonstrates the stability of the Protochips Fusion system at high temperatures. This video was taken on a JEOL ARM200F (200 kV, Cs aberration correction) at JEOL in Akishima, Japan. For more information on Fusion, visit www.protochips.com/fusion
An Fe nanoparticle etching few-layer graphene (FLG) at 900 degrees C and 600 Torr of H2. The Fe nanoparticle preferentially etches the graphene along specific crystallographic directions.
Credit: SI-IPCMS-CNRS/University of Strasbourg, France: G. Melinte, S. Moldovan and O. Ersen
The Fusion™ heating and electrical biasing system is compatible with environmental electron microscopes. This real time video shows ceria (CeO2) in a reducing atmosphere of hydrogen at 1.2 Torr at 750 C. The lattice and surface reactions can be easily seen.
This real time video shows two gold nanoparticles that are on the surface of larger iron oxide particles at 900 C. At this temperature the gold is very mobile, and the two particles coalesce into one larger nanoparticle. This demonstrates the stability of the Protochips Fusion™ system at high temperatures. This video is in real time and taken on a JEOL ARM200F (200 kV, Cs aberration correction) at JEOL in Akishima, Japan.
#FindYourBreakthrough | FLASH TALKS: EP #3
Iron-silica Interaction During Reduction of Precipitated Silica-promoted Iron
Oxides Using In Situ XRD and TEM
Presented by: Dr. Jaco Olivier from Nelson Mandela University. Read the full publication here: https://www.sciencedirect.com/science/article/abs/pii/S0926860X21000454?via%3Dihub
#FindYourBreakthrough | FLASH TALKS: EP #6
Dynamic Restructuring of Supported Metal Nanoparticles and its Implications for Structure Insensitive Catalysis
Presented by: Dr. Charlotte Vogt from Technion – Israel Institute of Technology. Read the full publication here:
https://www.nature.com/articles/s41467-021-27474-3
#FindYourBreakthrough | FLASH TALKS: EP #7
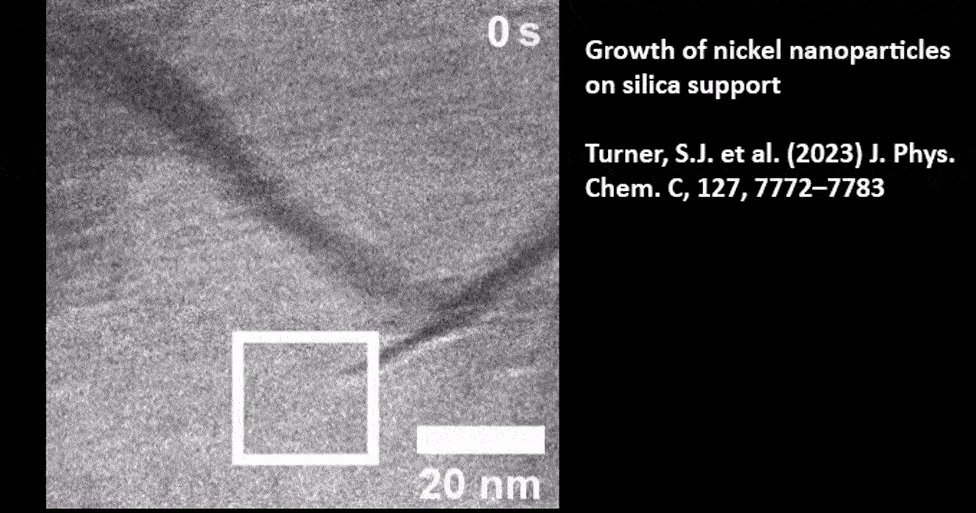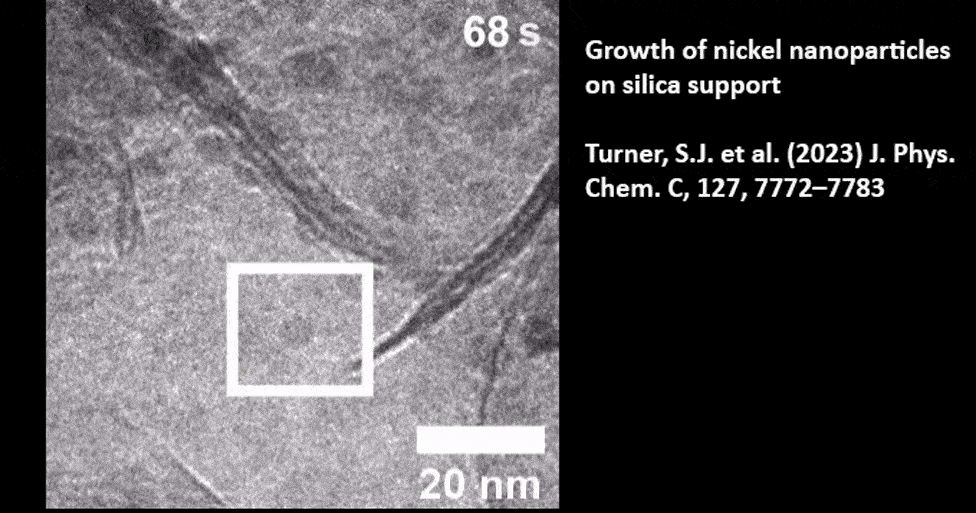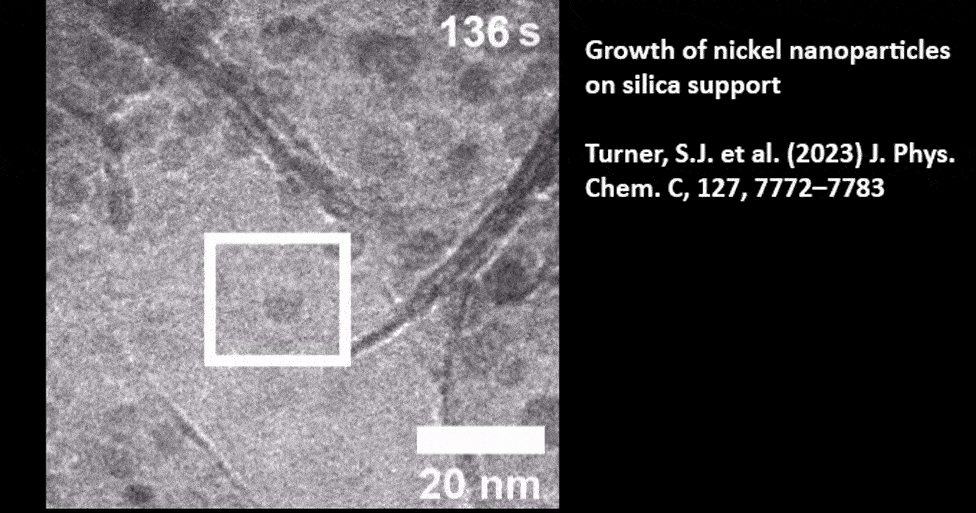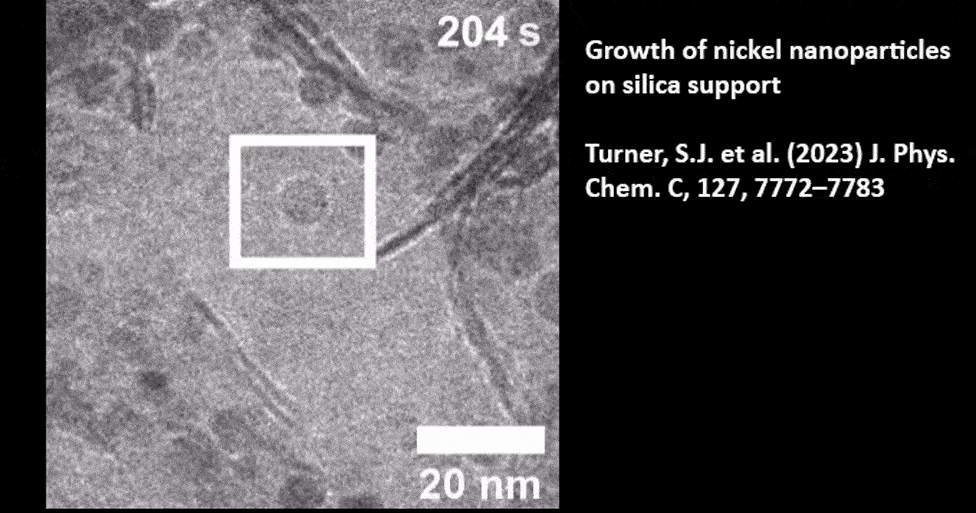
Catalyst Particle Growth at the Nanoscale
In Situ TEM of TiO2 Supported Gold Nanoparticle Growth
Presented by: Dr. Mark J. Meijerink from Utrecht University. Read the full publication here:
https://pubs.acs.org/doi/10.1021/acs.jpcc.9b10237
#FindYourBreakthrough | FLASH TALKS: EP #8
Attachment of Iron Oxide Nanoparticles to Carbon Nanofibers Studies by In-Situ Liquid Phase Transmission Electron Microscopy
Presented by: Dr. Nynke A. Krans from Utrecht University. Read the full publication here: https://pubmed.ncbi.nlm.nih.gov/30468967/
#FindYourBreakthrough | FLASH TALKS: EP #
Formation mechanism of high-index faceted Pt-Bi alloy nanoparticles by evaporation-induced growth from metal salts
Presented by: Dr. Kunmo Koo from Northwestern University Atomic and Nanoscale Characterization Experimental (NUANCE) Center. Read the full publication here: https://www.nature.com/articles/s41467-023-39458-6
Products featured: Protochips Atmosphere AX gas cell system for TEM with AXON machine vision software
#FindYourBreakthrough | FLASH TALKS: EP #17
High-Entropy-Alloy Nanocrystal Based Macro- and Mesoporous Materials
In this flash talk, a soft-chemistry route to fabricate ordered macro- and mesoporous materials based on high-entropy alloy nanoparticles is introduced. In situ electron microscopy is used to observe the stability of these newly formed nanoparticles under atmosphere.
Presented by: Dr. Maria de Marco from the Laboratoire de Chimie de la Matière Condensée de Paris (LCMCP) and the Institut de Physique et Chimie de Matériaux de Strasbourg (IPCMS). Read the full publication here:
https://pubs.acs.org/doi/10.1021/acsnano.2c05465
Products featured: Protochips AtmosphereAX
In this flash talk, Dr. Abdallah Nassereddine from the Université Cité Paris talks about atomic-scale in situ TEM using the Atmosphere AX system. This research is combined with DFT-based calculations to explore the reactivity of TiO₂-supported gold nanoparticles (below 7nm) with 1,3-butadiene. This study reveals the dynamic structural evolution of these nanoparticles at 300°C and 200°C, highlighting significant morphological changes and adsorption behaviors.
Presented by:
Abdallah Nassereddine, PhD.
Université Cité Paris, France
Read the full publication here:
https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/cctc.202300434
This study tackles a major issue in converting methanol into useful products: the catalyst used in the process tends to wear out quickly due to the buildup of carbon deposits, known as coke. Dr. Sharmin Sharna and collaborators discovered that adding a small amount of liquid gallium, a metal that melts at low temperatures, to a zeolite catalysts, ZSM-5, dramatically improves its durability. The study added methanol in vapor form in the Atmosphere AX system to observe the reduction of carbon buildup on the catalysts' surface. Adding gallium facilitated the removal of carbon, allowing the catalyst to last up to 14 times longer than the original version! This breakthrough could lead to the development of longer-lasting catalysts for the methanol-to-hydrocarbons process, making it more efficient and cost-effective.
Presented by :
Sharmin Sharna, PhD
IPCMS in collaboration with UCCS
Read the full publication here:
https://www.nature.com/articles/s41467-024-46232-9